

[Crafts] Using salt!? Let’s Draw Sparkling Pictures!
Crafts
Using salt!?Let’s Draw Sparkling Pictures!
In this "Let's Think!" section, we'll have a quiz about how salt dissolves in water. How many questions can you answer?
What You'll Learn in "Let's Think"
- Does salt dissolve in water?
- Does the temperature of water affect how salt dissolves?
- What happens when saltwater dries up?

Do you know that salt dissolves in water?

Seawater tastes salty because salt is dissolved in it.

That's right.
When you put salt in water, it starts to dissolve.
When you put salt in water, it starts to dissolve.

In the next video, you can watch salt dissolving in water. See that cloudy swirl just under the net? That’s the salt starting to dissolve.

Wow, that's so cool!

Alright, it's quiz time!

I'm great at quizzes!

Alright, here's the first question.
How much salt do you think can dissolve in one 500ml bottle of water?
How much salt do you think can dissolve in one 500ml bottle of water?


I have no idea!

That's a tough one... Just take your best guess.

Hmm... I'll pick number 2.

Nope. Sorry, the correct answer is number 3. It's 180 grams. Was that more than you expected? That’s a lot of salt!


What happens if you add even more?

Shall we try it?
Wait, even if you stir, the salt just sinks to the bottom.
When no more salt can dissolve, it’s a slightly difficult word, but we call that state "saturation."
Wait, even if you stir, the salt just sinks to the bottom.
When no more salt can dissolve, it’s a slightly difficult word, but we call that state "saturation."


Okay,for the second question,
which can dissolve more salt: hot water or cold water?
which can dissolve more salt: hot water or cold water?
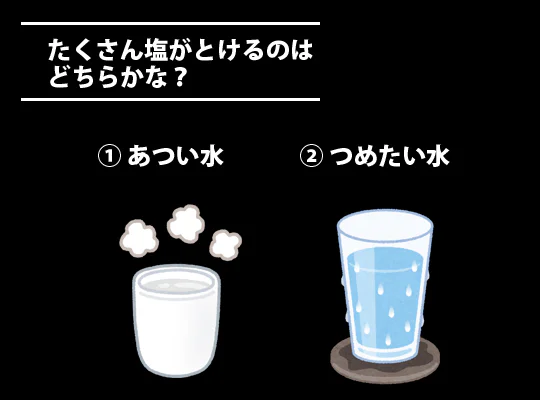

Hot water?

Correct!
With the same amount of water, warmer water can dissolve more salt.
With the same amount of water, warmer water can dissolve more salt.


Okay, here's the final quiz.
If you take saltwater that's in the "saturated" state and let all the water evaporate, what happens?
If you take saltwater that's in the "saturated" state and let all the water evaporate, what happens?

Hmm... Well, If the water’s gone, the salt will come back?

Correct again! The salt "crystals" that had dissolved in the water will reappear. This is called "recrystallization."。



Questions 2 and 3 were easy, weren't they?

Even though they might seem easy, they're very important, so make sure you remember them!

Alright, it's finally time for our "Let's Try" section.
What You Learned in "Let's Think"
- When no more salt can dissolve, we call that state "saturation."
- When the water temperature is higher, more salt can dissolve.
- When salt recrystallizes, you can see it with your own eyes.